Large amounts of soluble manganese(III) identified at the land-ocean interface
Soluble manganese(III) [Mn(III)] is a phase traditionally considered non important in aqueous redox reactions. However, applying a spectrophotometric method sensitive to Mn oxidation state, Madison and co-authors found that up 90% of the Mn in porewater collected from sediment cores in the St. Lawrence Estuary is soluble Mn(III). The authors conclude that the conceptual model of the sedimentary redox cycle should be reviewed to include dissolved Mn(III). This result also has implication for the oxidation of iron(II) [Fe(II)] to Fe(III) process and, because Mn(III) can act as either an electron acceptor or an electron donor, the authors suggest that reduction-oxidation capacity of the soluble Mn pool in sediments has been underestimated.
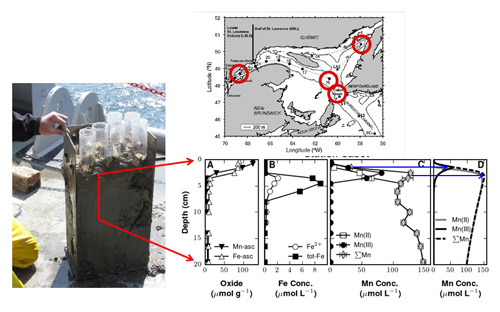
Figure: This figure shows the study sites (on the top), a picture of a core (on the left) and Mn and Fe profiles. Picture © George W. Luther III. Please click here to view the figure larger
Mn(III) can also form when MnO2 oxidizes Fe(II) to Fe(III). Figure A shows the solid phase data; Figure B the soluble Fe data; Figure C the soluble Mn data; in Figure D, a diagenetic model was used to profile the data. The agreement is good as various reactions for the production and loss of Mn(III) were added to the model. Models can finally predict the position and shape of all soluble Mn species.
Reference:
Madison, Andrew S., Bradley M. Tebo, Alfonso Mucci, Bjørn Sundby, and George W. Luther III (2013) Abundant Porewater Mn(III) is a Major Component of the Sedimentary Redox System, Science 23 August 2013: 341 (6148), 875-878. DOI:10.1126/science.1241396
