Sedimentary controls on seawater nickel distributions and nickel isotope compositions: a two steps study
Nickel (Ni) isotopic mass balance in the ocean stands among the less understood so far. To make it simple, a significative source of heavy Ni is missing. Chen and colleagues (2024 and 2025, see references below) tackled this issue by conducting a detailed study of the Ni behaviour associated with manganese (Mn) mineral transformations during the sediment diagenetic processes.
In their first study in 2024, they established the speciation of Ni species during these processes. The underlying concept is that, because bonding strength governs equilibrium stable isotope fractionation, the differences in bonding strength of Ni species to phyllomanganate suggest that different Ni species might have different isotope behaviours during adsorption. Indeed, this first study demonstrates that Ni adsorbed in the presence of organics should be isotopically light relative to Ni adsorbed in the absence of organics, results consistent with the heavy Ni isotope composition of Fe-Mn crusts (low TOC content) while Mn-rich sediments (high TOC content) are isotopically light.
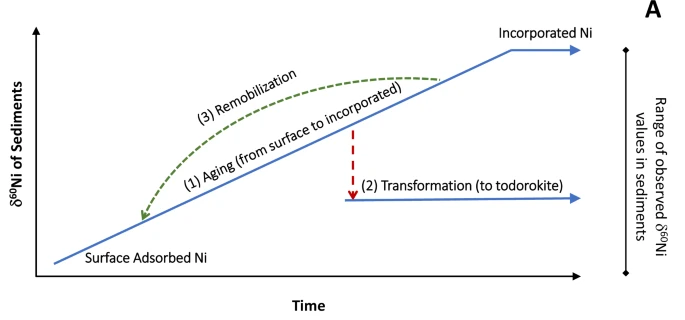
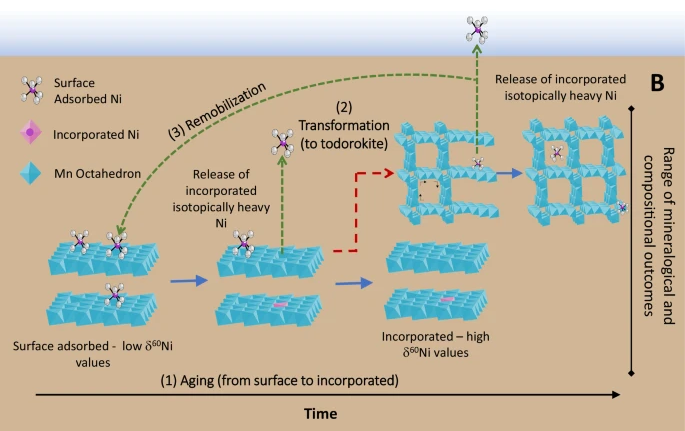
In a subsequent study in 2025, the same authors established that the burial of Ni in marine sediments exerts a primary control over the oceanic concentration and isotopic composition of Ni. Actually, the “two bonding mechanisms speciation” described above evolves during Mn mineral aging and that isotopically heavy Ni is preferentially released during this transformation. In other words, Mn mineral aging and transformation is modifying both the sediment and seawater Ni isotopes. This finding leads to the conclusion that sediment diagenesis releases isotopically heavy Ni to porewaters, which could be returned to bottom waters: the deep ocean would be thus enriched in heavy Ni, one of the Ni oceanic “missing source”.
References:
Chen, L., Downey, A. R., Archer, C., Little, S. H., Homoky, W. B., & Peacock, C. L. (2025). Mineralogical controls of the oceanic nickel cycle. Nature Communications, 16. Access the paper: 10.1038/s41467-025-62842-3
Chen, L., Homoky, W. B., & Peacock, C. L. (2024). Speciation controls on Ni adsorption to birnessite and organo-birnessite. Chemical Geology, 654, 122067. Access the paper:10.1016/j.chemgeo.2024.122067
