An original approach to assess the particulate trace metal concentrations in seawater
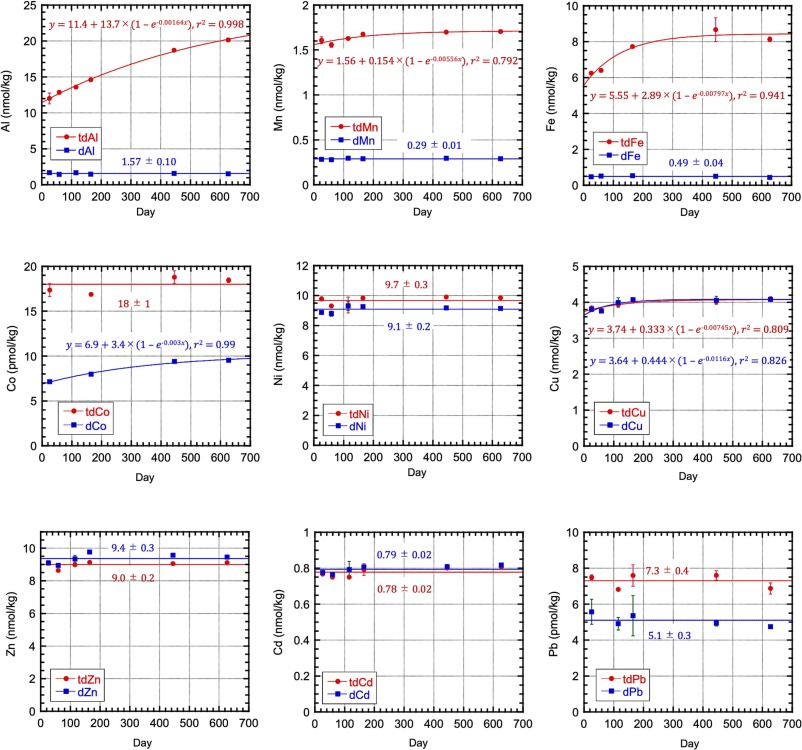
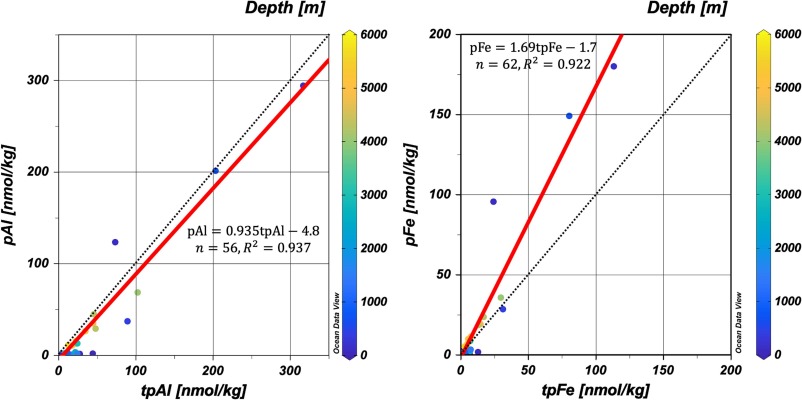
Measuring the particulate trace metal (pM) concentrations in seawater is challenging, even though this parameter is essential to understand the trace metal cycles, a GEOTRACES mission. Sohrin and co-workers (2025, see reference below) propose defining pM as the difference between total dissolvable and dissolved metals after a long storage (approximately two years) of filtered and unfiltered seawater acidified to pH 1.9. They observed that pM increased in two stages and reached equilibrium within ~ 1 y for relatively soluble elements as cobalt (Co) and lead (Pb), and took twice as long for aluminium (Al) and iron (Fe). They then compared their results with those obtained by a strong-acid leaching of filtered particles (total particulate metal; tpM) and established that pM determined by their protocol is a good proxy for tpM. Although they acknowledge some drawbacks for their approach -mostly related to the occurrence of refractory minerals in the samples- they consider that, if researchers are patient enough, they can assess a proper concentration of pM via the proposed protocol: pM result is related to the intercalibrated concentration of dM; approximately a hundred grams of filtered and unfiltered seawater subsampled from a sampling bottle are sufficient to determine pM; the risk of contamination is low because strong acids at high concentrations are unnecessary.
Reference:
Sohrin, Y., Zheng, L., Chan, C.-Y., Nakaguchi, Y., Takano, S., Sohrin, R., Liao, W.-H., & Ho, T.-Y. (2025). Acid-leachability of metals from suspended particles in the Pacific Ocean. Marine Chemistry, 273, 104571. Access the paper:10.1016/j.marchem.2025.104571
